Genomic patterns of malignant peripheral nerve sheath tumor (MPNST) evolution correlate with clinical outcome and are detectable in cell-free DNA
Isidro Cortes-Ciriano*1, Christopher D. Steele2, Katherine Piculell3, Alyaa Al-Ibraheemi4, Vanessa Eulo5, Marilyn M. Bui6, Aikaterini Chatzipli7, Brendan C. Dickson8, Dana C. Borcherding9, Andrew Feber10, Alon Galor11, Jesse Hart12, Kevin B. Jones13, Justin T. Jordan14, Raymond H. Kim15, Daniel Lindsay16, Colin Miller1, Yoshihiro Nishida17, Paula Z. Proszek10, Jonathan Serrano18, R. Taylor Sundby19, Jeffrey J. Szymanski20, Nicole J. Ullrich21, David Viskochil22, Xia Wang23, Matija Snuderl18, Peter J. Park7, Adrienne M. Flanagan*2,16, Angela C. Hirbe*9, Nischalan Pillay*2,16 and David T. Miller*3; for the Genomics of MPNST (GeM) Consortium Affiliates.
Author affiliations can be found in the full article online (linked below)
Abstract
Malignant peripheral nerve sheath tumor (MPNST), an aggressive soft-tissue sarcoma, occurs in people with neurofibromatosis type 1 (NF1) and sporadically. Whole-genome and multiregional exome sequencing, transcriptomic, and methylation profiling of 95 tumor samples revealed the order of genomic events in tumor evolution. Following biallelic inactivation of NF1, loss of CDKN2A or TP53 with or without inactivation of polycomb repressive complex 2 (PRC2) leads to extensive somatic copy-number aberrations (SCNA). Distinct pathways of tumor evolution are associated with inactivation of PRC2 genes and H3K27 trimethylation (H3K27me3) status. Tumors with H3K27me3 loss evolve through extensive chromosomal losses followed by whole-genome doubling and chromosome 8 amplification, and show lower levels of immune cell infiltration. Retention of H3K27me3 leads to extensive genomic instability, but an immune cell-rich phenotype. Specific SCNAs detected in both tumor samples and cell-free DNA (cfDNA) act as a surrogate for H3K27me3 loss and immune infiltration, and predict prognosis. SIGNIFICANCE: MPNST is the most common cause of death and morbidity for individuals with NF1, a relatively common tumor predisposition syndrome. Our results suggest that somatic copy-number and methylation profiling of tumor or cfDNA could serve as a biomarker for early diagnosis and to stratify patients into prognostic and treatment-related subgroups.
Data availability
Raw sequencing data has been deposited at the European Genome-phenome Archive (EGA), which is hosted by the EBI and the CRG, under the dataset accession number EGAD00001008608. Ultra-low-pass whole-genome sequencing data of cell-free samples is available under controlled access data at https://doi.org/10.7303/syn23651229.
Correspondence and requests for materials should be addressed to David T. Miller (MD, PhD): david.miller2@childrens.harvard.edu
Code availability
The code used to process the raw data is available upon request.
We are pleased to share GeM Consortium data with domestic and international research groups. Here is where the data is currently being shared:
United States of America (20 researchers)
Canada (1 researcher)
Spain (2 researchers)
United Kingdom (6 researchers)
France (2 researchers)
Germany (1 researcher)
Australia (1 researcher)
The GeM Consortium represents the most thorough and intensive classification of MPNSTs. Our results suggest that accurate clinical pathology classification requires incorporation of genomic information.
In all cases, the early events in MPNST evolution involve biallelic inactivation of NF1, TP53 and CDKN2A, as well as mutations in PRC2 complex genes in a subset of cases.
We looked for possible targets of therapy like immune signature and gene fusions (has not been studied previously), and were not able to identify these potential targets
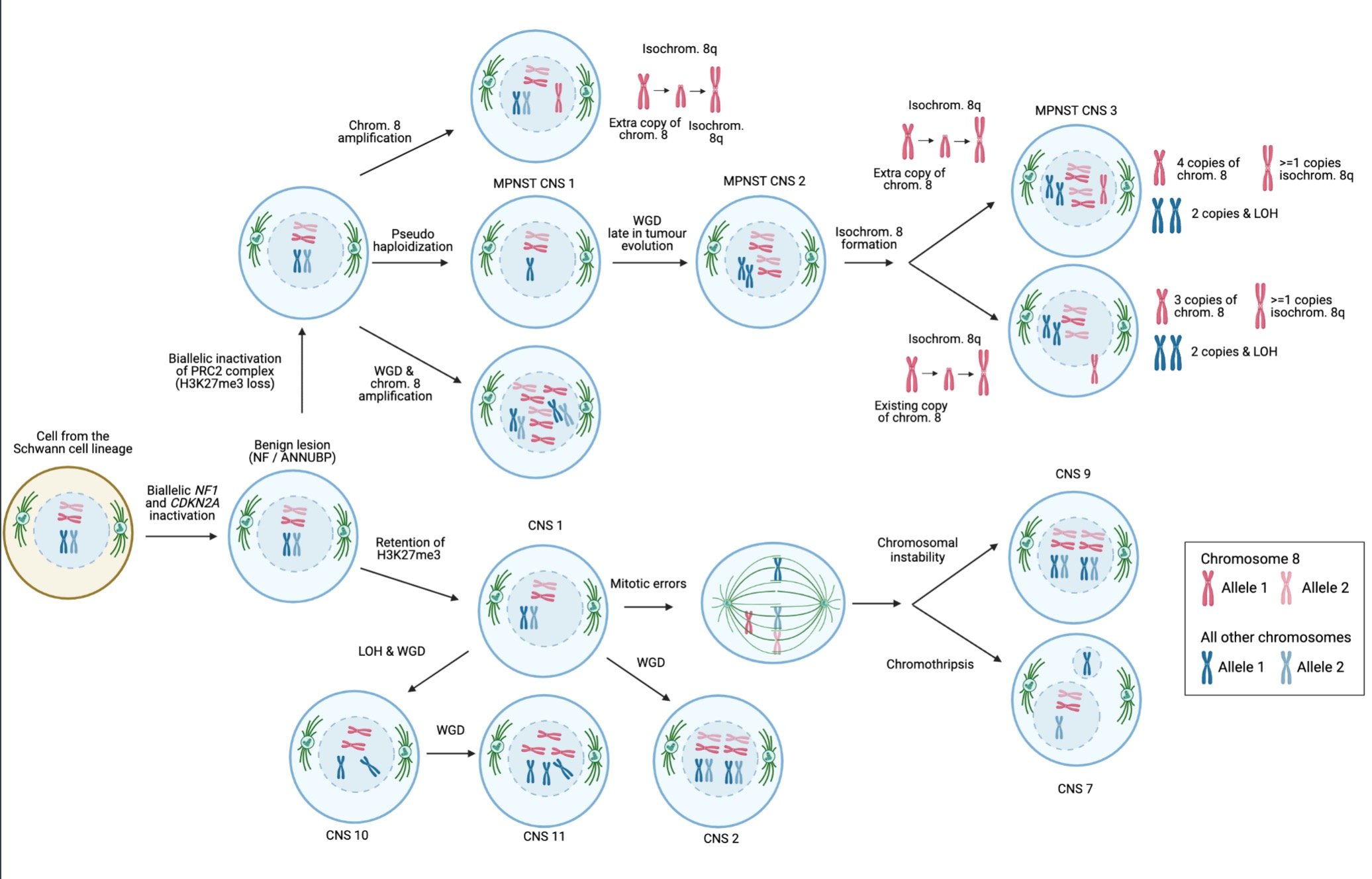
Our detailed analysis of the genomic architecture revealed distinct pathways of tumor evolution that can be identified through H3K27 methylation status.
Pathways of MPNST evolution. Schematic representation of the order and timing of events involved in the evolution of MPNSTs. Copy number signatures predominant in specific karyotypic configurations are shown. CN, copy number signature; chrom., chromosome; isochrom., isochromosome; PRC2, polycomb repressive complex 2; WGD, whole genome doubling.
The NF Research Initiative at Boston Children's Hospital seeks to transform therapeutic development of NF-1 related Malignant Peripheral Nerve Sheath Tumors (MPNST) through international collaboration to improve the biological understanding of tumor progression in this disorder.